You may not realize it, but you’re already driving a solar-powered car. While they no longer resemble a ray of light, the chemical bonds contained within a gallon of gasoline are filled with solar energy captured millions of years ago by plants. All plants use water and sunlight to capture and convert carbon dioxide (CO2) into more useful forms of carbon in a process known as photosynthesis. When we burn gasoline in an engine, we’re breaking these bonds to regenerate CO2 and reclaim the stored energy from the sun. This process has powered our planet for the last century, but the release of hundreds of millions of years’ worth of stored CO2 is inherently unsustainable and has begun to dramatically alter our climate. How can we continue to drive, fly and power our cities without blanketing our planet with CO2?
Just like plants use sunlight and carbon dioxide to create their own food, the Lewis Lab at Caltech is trying to use sunlight and water to produce fuel and oxygen.
Under the supervision of Professor Nate Lewis at Caltech, my research group is working towards creating a device which converts water and sunlight directly into a fuel that can fill up your gas tank or power your home. Although we’re inspired by the photosynthesis accomplished by nature, we need to work quicker and more efficiently than plants in order to compete with the low cost of burning natural gas and the high efficiency of storing electrical power in batteries. Natural photosynthesis uses two separate light absorbers and a catalyst—a material which speeds up a chemical reaction—to capture energy from sunlight. This energy is used to pull apart water molecules, forming oxygen and protons. These protons are used to produce molecules that convert CO2 into the glucose and starch that the plant uses for energy when the sun isn’t shining. In contrast, artificial photosynthesis skips most of these intermediate steps by directly converting water into oxygen and hydrogen gases. Hydrogen can be used directly as a clean fuel which produces nothing but pure water vapor.
To do this, we’ve replaced nature’s toolbox with more efficient and longer-lasting modern materials. Our devices absorb sunlight with tiny hair-like structures made from semiconductors, such as silicon, the same materials used to produce commercial solar panels. These light-absorbers are embedded in a thin sheet of plastic, so instead of resembling a bulky solar panel, our devices are as light and flexible as silk. Just like plants, we pair our light-absorbing materials with a catalyst in order to quickly use solar energy for chemical reactions before that energy is lost as waste heat. By collecting more sunlight and driving faster chemical reactions, research groups around the world are beginning to demonstrate that devices for artificial photosynthesis can convert sunlight and water into fuel at an efficiency ten times that of plants. We’re moving closer to our dream of an artificial grass which could provide fuel to power our cars, but there are still major scientific and engineering challenges which remain unaddressed.
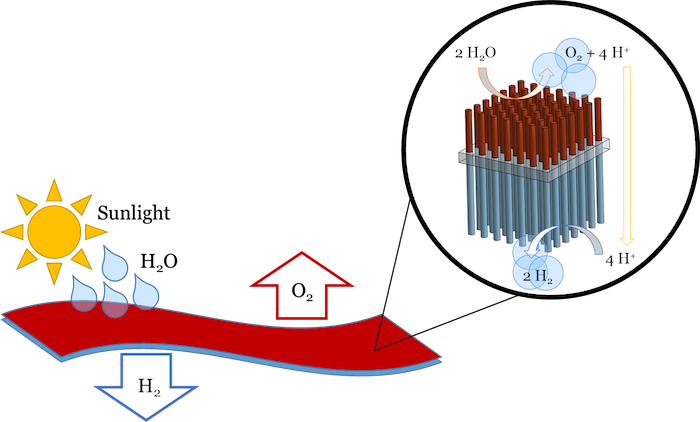
Artificial turf could convert water and sunlight into fuel. Tiny wires collect sunlight and use the energy to split water into hydrogen gas (a fuel) and oxygen (the byproduct). For stability and safety, a flexible membrane separates the two gases being produced.
Paul Kempler
The primary roadblock to our dream of artificial photosynthesis is bringing together the components in a way that ensures industrial-scale devices are cost-effective and can operate without assistance for a decade or more. Now that high-efficiency devices for converting sunlight and water into a fuel have been demonstrated, we also need to show that these devices can be built and installed cheaply over large areas. The installation and wiring of bulky solar panels is time-consuming and expensive. An ideal solar-to-fuels device would be wireless, and passively produce fuels for storage. My research group is tackling this problem by designing fully integrated devices which could operate without the use of external wires and could direct the flow of hydrogen and oxygen using structures inspired by nature.
Instead of working with conventional, large, flat panels for our light absorbers, we’re researching microwire and nanowire light absorbers, which require less material to absorb the same amount of sunlight. This means that they could eventually become less expensive to produce. These wires are even smaller than the tiny hairs on the inside of your ear, but they are the workhorse of the device. Microwires can be embedded into a stretchy piece of plastic so that they’re flexible and sturdy but still perform the same job of a conventional solar panel. In this form, installation of our device could be as simple as rolling out a large tarp onto a sunny plot of land. Although the devices we test in our labs are often smaller than your thumbnail, one day they’ll need to be the size of football fields if we want to meet the demands of our energy-hungry society. During the day, the top of these fields would exhale oxygen into our atmosphere while hydrogen fuel would be collected beneath the turf.
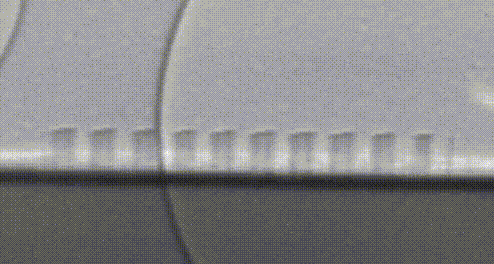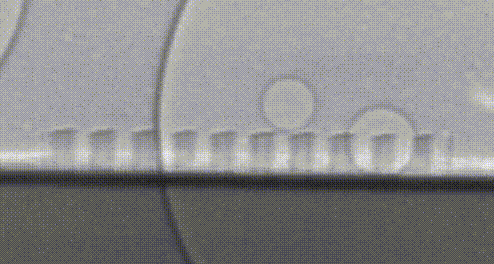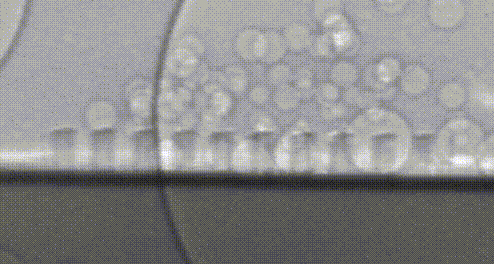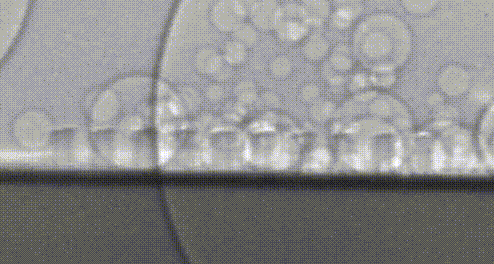
High-speed video of hydrogen bubbles being generated on a silicon microwire array.
Professor Rob Coridan, University of Arkansas
Collecting these valuable hydrogen bubbles is an often-overlooked challenge. If everything worked perfectly, all of our bubbles would naturally float away from the surface—but bubbles are sticky. Picture the last time you poured a fizzy drink and how the bubbles clung to the side of the glass. In our devices, these bubbles act like a clog in a drain and prevent the flow of electrical current so that we can’t continue to make fuel. Recently, my research has focused on how we can control the collection of fuel beneath this turf without the need to pump or stir these bubbles—further simplifying our design and making it less expensive to install. Once again, we’re taking inspiration from nature, and structuring our light absorbers to resemble surfaces in nature which repel water droplets. A duck structures its feathers with precisely spaced micro-hairs which force water to bead up and roll away, allowing the bird to stay dry. We noticed that the size and structure of our microwires could be leveraged to force bubbles away in a similar manner, preventing the bubbles from clogging up fuel production. Now we’re using real-world prototypes, computer simulations, and high-speed photography to understand how we can better understand and improve this behavior.
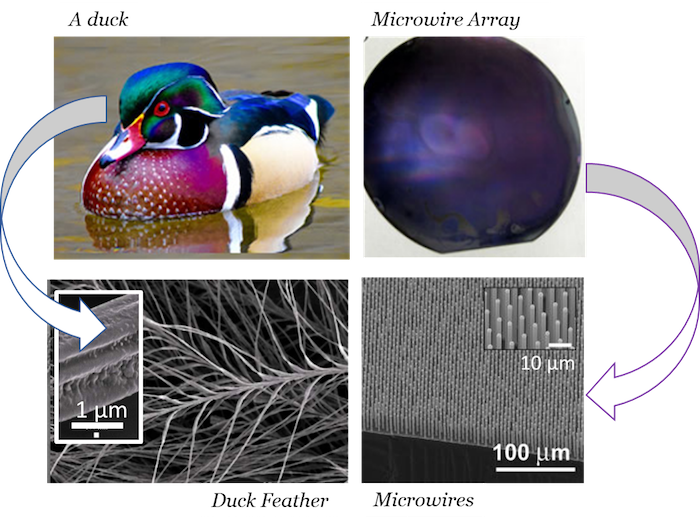
Silicon microwire arrays resemble duck feathers due to the presence of tiny features that scatter light. This microstructure is also what allows our microwires to repel water—like water off a duck's back. 1 µm is 0.000001 meters, or one millionth of a meter.
Paul Kempler. Lower left inset adapted from Y. Liu et al. (2008). Wire array photo courtesy of Adele C. Tamboli et al. (2012).
Driving through the California desert, I’m inspired by how quickly solar panels and wind turbines have covered the otherwise arid landscape to produce energy from renewable resources. I dream of a day when some of this land could be covered with artificial turf, quietly producing fuels for our cars and oxygen for the air we breathe. I believe a carbon-neutral future is within our reach, and can be achieved without having to give up the cars and planes we’ve become reliant on. If we can learn from plants, we’ll do this with water and sunlight, so that we can forever close the carbon cycle and keep our planet healthy for future generations.

Bill Gates visited our lab in the fall of 2016 to hear about our research on solar fuels. Here, my advisor and I are showing him how we grow our silicon microwires.
The Gates Foundation




