Have you ever wondered where the particles we are made of come from? In the words of the late, great Carl Sagan, ‘We are made of star stuff.’ The hydrogen and oxygen in water, the carbon in every life form, the nitrogen in our DNA, the calcium in our bones and teeth, the iron in our blood, and the gold we wear were all made in exotic places in the Universe. To understand how this came to be, we need to zoom out of your room, city, and even Earth and take a journey to the edge of the Universe.
Before starting this journey, we will detour to some basic science. What makes carbon, oxygen, hydrogen, and all other elements different? To answer this question, we have to take a look inside the structure of the elements. The constituent particles of an element are called atoms, which are the smallest building blocks of all matter. Atoms are made of positively charged protons and chargeless neutrons packed tightly into a highly compact nucleus that is orbited by negatively charged electrons. The number of protons in the nucleus determines the elemental identity of the atom. For example, eight protons combine with eight neutrons and eight electrons to create oxygen, twenty of each combine to form calcium, and so on. From a pool of a little over one hundred different elements comes your body and everything we know on Earth. But where and how did these elements, the basis for our entire existence, originate?
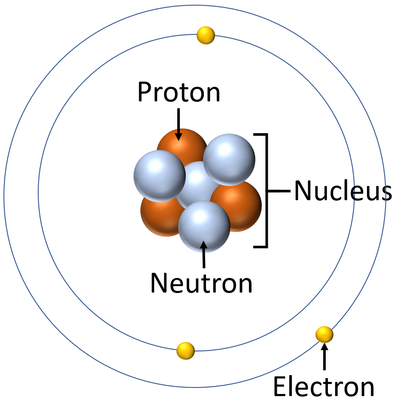
Schematic of an atom showing the general arrangement of protons, neutrons, and electrons
Let us start with the simplest element, hydrogen, which is made of one proton in the nucleus and one orbiting electron. Hydrogen is present in the water we drink, the food we eat, and the fuels we put in our cars. To see where hydrogen first formed, we need to travel back to the start of time, to the birth of the Universe itself. Astronomers have theorized that the Universe began with the Big Bang—an explosion with extremely high energy. Immediately after the Big Bang, bits and pieces of matter and energy rapidly expanded into the Universe. In these early moments, however, the environment was far too hot, dense, and chaotic for larger structures—like atoms and molecules—to form. Hydrogen didn’t appear until the Universe had spread out and subsequently cooled down—enough for the first protons, neutrons, and simple atoms to form. Electrons were formed within the first microsecond (one-millionth of a second) after the Big Bang. Protons and neutrons began forming shortly after, within a single second. About three minutes after the Big Bang, conditions had cooled enough for these protons and neutrons to form a hydrogen nucleus. This is called the era of nucleosynthesis (i.e., the synthesis of atomic nuclei). As the Universe cooled down further, these hydrogen atoms came together under the influence of gravity to form the first stars of the Universe. Continuing our journey to understand the basis of our existence, we now turn to these twinkling stars.
Humanity has looked at the stars and wondered about our place under them for hundreds of years. In the bellies of these stars—seemingly serene dots in the sky—a high-pressure inferno begins to squeeze together atoms of lighter elements and combine them into heavier ones. Because all protons are positively charged, they tend to repel each other. However, the extreme conditions of stellar interiors make them one of the few places in the Universe where protons can be forced to fuse into a heavier nucleus. Let’s examine this fusion process more closely. When two protons and two neutrons are squeezed together, they form the nucleus of helium. This nuclear fusion process releases enough energy to power the Sun. Then, three helium nuclei can combine in a multi-step process to form the nucleus of carbon, a 6-proton element. The fusion of carbon and helium nuclei leads to an oxygen nucleus containing 8 protons. The process continues. Through successive fusion reactions, the nuclei of most elements lighter than iron (26 protons) can be formed. The atomic factories inside stars continuously churn day in and out, generating all the carbon and oxygen atoms in the Universe. In fact, all the organic elements inside plants, animals, and even humans once came from the bellies of stars.
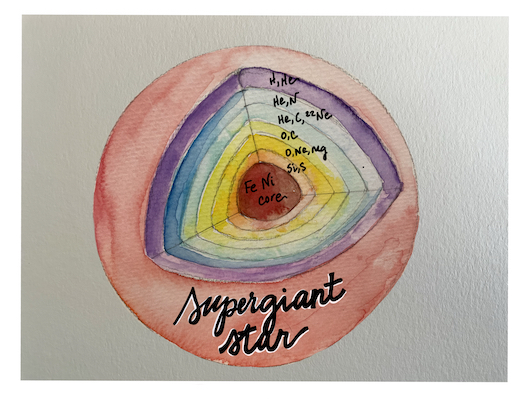
The extremely high pressures and temperatures in the interiors of stars provide ideal conditions for the generation of atoms of elements like carbon and oxygen.
Illustration by Sarah Zeichner for Caltech Letters
While lighter elements are created in living stars, heavier elements originate in magnificent, explosive stellar deaths. When a star dies, it explodes in a phenomenon known as a supernova, an explosion so bright that it may even look like a second sun in the sky. In the few seconds of a supernova, more energy is released than during a star’s billion-year lifetime. In 1054 AD, Chinese astronomers discovered such a second ‘sun’ visible in the daylight for nearly twenty days. This energy provides ideal conditions for heavy atomic nuclei to bind together and form even heavier elements, such as calcium and iron. My own research involves scanning the night sky to discover new supernovae. We then study how they evolve over time with the hope of understanding the physics driving the explosion itself, and the processes involved in making these heavy elements.

An artistic rendering of a supernova
Illustration by Sarah Zeichner for Caltech Letters
There are much heavier elements of course, like gold, that even supernovae can’t create. So where do these elements form? You might understand the game by now: we need to travel to places in the Universe that are even more exotic. After a supernova explosion, what’s left is an extremely high-density object called a neutron star. It’s so dense that a spoonful of a neutron star weighs more than the Himalayas! These highly compact objects provide a gigantic reservoir of neutrons essential for the synthesis of heavier elements. But to access this neutron reservoir, we need a way to tear it open. In this pursuit, we now turn to binary neutron stars. While we started our journey in a single star, like our Sun, most stars are actually binary (double): two stars orbiting each other. After both stars in a binary system explode, the two dense neutron stars left behind eventually spiral into each other and collide, releasing neutrons heated to billions of degrees. Heavy elements form in under a second. In 2017, observations made by Caltech scientists and others helped astronomers discover such a merger for the first time. The color and brightness of the merger provided direct evidence for the presence of gold and platinum—it was like observing alchemy in action!
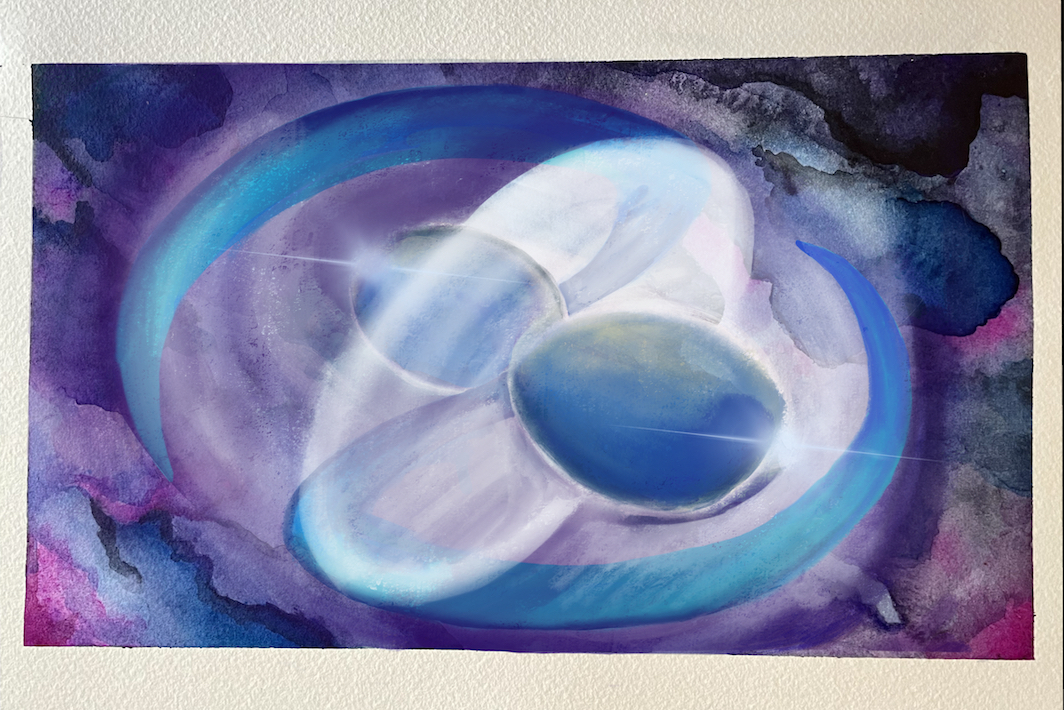
Two neutron stars spiral in moments before merging, resulting in a highly energetic explosion that created the first gold and platinum atoms.
Illustration by Sarah Zeichner for Caltech Letters
How did these elements, formed billions of years ago, finally make their way to us? As stars die and explode as supernovae, they release all the elements they made into space. Similarly, mergers of stellar corpses also produce explosions that expel all synthesized elements, forming a cloud of gas and dust. This stardust later forms new stars, like our Sun, and new planets, like Earth. Astronomers believe that our solar system formed from one such cloud of stardust called the Solar Nebula around 5 billion years ago.
The origin story of the atoms that make up our muscles, bones and blood, the jewelry we wear, and the smartphones we use, a story that predates us by billions of years, is identical for each and every one of us. It is a story independent of one’s caste, race, religion, or country. Times are uncertain. No matter what you are going through, remember that we all are connected by this incredible, better-than-science-fiction story. You are made of stardust; every bit of you is glorious star stuff. Go out there and shine!